2. Construction of twenty elements system on the dodecahedron
Before constructing the
model using the side chains of amino acids, was built a prototype, the
structure of the 20 abstract elements arranged in a dodecahedron. Symbolically
elements are denoted by letters of the Latin alphabet and numeric indexes.
2.1. Properties of the
dodecahedron and the allocation of the planes of antisymmetry in it
|
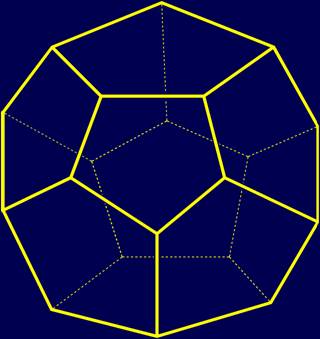
Dodecahedron (Fig. 2) - one of the Platonic bodies. It has 20 vertices, 12 faces and 30 edges. |
Through
dodecahedron vertices it is
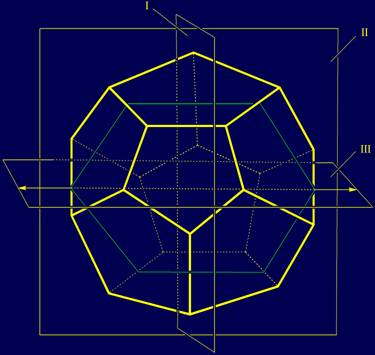
possible to lead 15 planes. For our construction,
however, we chose three mutually perpendicular planes (Fig. 3), which are not
in all "correct" from a mathematical point of view. For our construction it there will be
anti-symmetry planes. |
|
|
|
|
Plane I (quasi-mirror-antisymmetry) is perpendicular to the plane of
the sheet and divide the dodecahedron into two equal parts: left and right. Plane II (not
mirror antisymmetry) is in the plane of the sheet and
share dodecahedron into two unequal parts: front and rear. Plane III (rotating antisymmetry) is perpendicular to plane I and
horizontal. It shares the dodecahedron into two
equal parts - the upper and lower, when rotating around the axis. |
|
Fig. 2. Dodecahedron |
Рис. 3. Three mutually perpendicular planes, which can be carried through the
structure of the dodecahedron. I - plane of a quasi-mirror
antisymmetry; |
|
2.2. Arrangement of elements on a dodecahedron: an
antisymmetry principle
|
Abstract elements are analogues of the
side chains of amino acids. They are
also 20.. The first two elements are A and B. Their antipodes are elements –A and –B. The remaining elements were identified
on the basis of the letters A and B with indices 1 and 2. For all items that
are on the right of the plane I, the indexes are written at the top left of
the letters: 1A, 2A,, –1A, –2A and 1B, 2B, –1B, –2B. For elements that are on the left of the plane I the same indices are
at the bottom right of the letters: A1, A2, –A1, –A2 and B1, B2, –B1, –B2. |
||
|
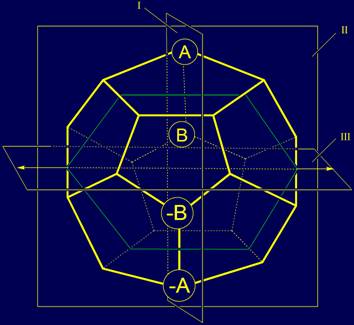
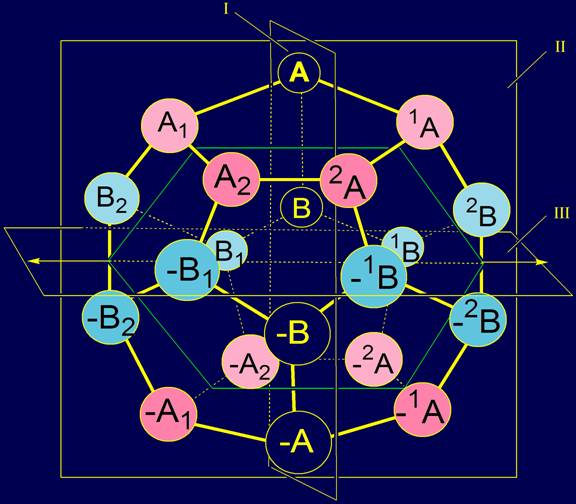
The elements of A, B,
and their antipodes –A and –B. Vertices, where there are elements A and B, and –A, –B, linked by edges. Elements-antipodes
are placed symmetrically relative to the plane III, and coincide when
rotating around the axis, lying in the plane III: A and -A, B and -B (fig.
4). |
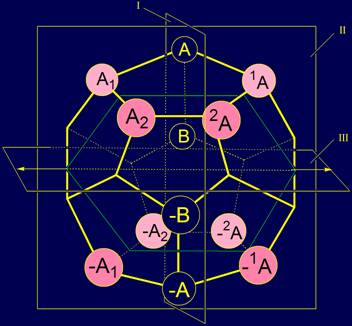
Elements 1A and A1 have been placed in
the vertices of the dodecahedron symmetrically to the right and to the left
of the plane I and the element A (Fig.
5), and elements A2 and A2 are also to the right
and to the left, but more far away from the element A, than the elements A1 and A1. Elements –1A,
–A1 and –2A,
–A2 will be located in relation to the plane I and the element –A
the same way. The arrangement
on a dodecahedron of all elements of type A is shown in Figure 5. |
Similarly arranged elements of type B. B1 and 1B were located to the
right and to the left of the plane I and B element . And the elements B2 and B2 were also placed to
the right and to the left, but more far away from the element B, than the
previous pair. Elements-antipodes –B1 and –1B, and –B2 and –B2 were placed on either
side of the plane I and element –B. The arrangement
on a dodecahedron of elements of type B is shown in Figure 6. |
|
Fig. 4. Location on the dodecahedron of elements A, B, -A, -B, which don’t have
antipodes respect to the planes I and
II . |
Fig. 5. The arrangement on a dodecahedron
of symmetric elements of type A. |
Fig. 6. The arrangement of elements of type B on
a dodecahedron. |
|
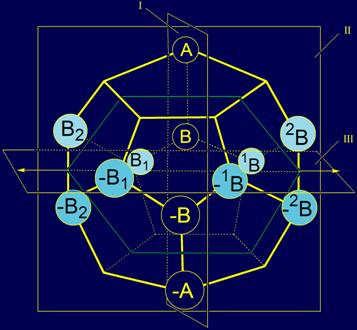
The whole system of 20 elements, located on
the dodecahedron is shown in figure 7. |
||
|
Fig. 7. The system of elements of type A and B on the dodecahedron. |
||
|
As a result we have received four subgroups
of elements: Two
subgroups of two elements:Subgroup 1: A and –A, a
subgroup 2: B and –B; Two
subgroups of 8 elements: Subgroup 3: 1A and A1, 2A
and A2, –1A
and –A1, –2A and–A2;
Subgroup 4: 1B and B1, 2B
and B2, –1B
and–B1, –2B and –B2. |
||
2.3. Transformations
of elements at transition through antisymmetry planes
Since we have three planes of
antisymmetry, then the transition through this plane to symmetric element may
be designated by any letter.
The following notation were introduced:
- Transition through a plane I - the letter a
(alpha),
- Transition through a
plane II - the letter b (бета);
- Rotation round an axis
laying in a plane III - the letter g (gamma).
Then it is possible to
describe all transformations of the elements, located in a column 1 in each
group, in the form of the following table.
|
|
1 |
a |
b |
g |
ab |
ag |
bg |
abg |
|
Subgroup 1 |
А |
|
|
- А |
|
|
|
|
|
Subgroup 2 |
B |
|
|
- B |
|
|
|
|
|
Subgroup 3 |
A1 |
1A |
A2 |
-A1 |
2A |
-1A |
-A2 |
-2A |
|
Subgroup 4 |
B1 |
1B |
B2 |
-B1 |
2B |
-1B |
-B2 |
-2B |
In
the process of further presentation, which is performed in section 3, Twenty side chains of amino acids were divided into four similar
subgroups which will be referred to as subgroup 1, subgroup 2, etc.
Address for connection: amino-acids-20@yandex.ru