4.2.3. Rotary antisymmetry
|
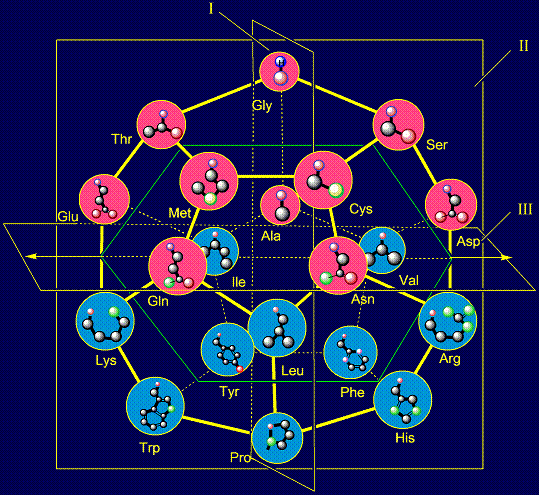
This type of antisymmety arises for two groups of side chains, which
are located above and below the plane III, separating the dodecahedron into
two parts: upper and lower. If the upper part of the dodecahedron, in which
the side chains of amino acids are
represented in the circles of pink, rotate around an axis in the
plane III, the vertices of the upper part coincide with the
vertices of the
bottom part of the dodecahedron, in which the side chains of amino
acids placed in the light blue circles. The resulting pair of side chains
are connected among themselves by transformation of rotation, which we denoted by the letter gamma (g).They have opposite properties and the different size. |
||||||
|
|
|
|
Unlike the previous types of anti-symmetry, which relate to the 8
pairs of side chains, this type of antisymmetry includes all 10 pairs of
amino acids. Two pairs of amino acids are located in the plane I. The first pair
is formed by glycine (Gly), which has no side chain - located above the plane
III (III (we will
speak further - above the plane), and proline (Pro - is under the
plane III), forming five-membered cycle built into the backbone of the
protein. It is shown in the first column on the right side. Their properties are opposite:
glycine has no side chain and do not affect the growth of alpha-helices,
whereas proline, forming a 5-membered ring, built in the main chain of a
protein, breaks its development. On the right are pairs of side chains of alanine (Ala - on the plane)
and leucine (Leu - under the plane III). They are shown in the third column
on the left side. Their properties are also opposite: alanine has the
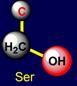
shortest non-polar side chain, and leucine – the longest one. Below on the right side of the dodecahedron a pair of serine (Ser -
above the plane) - histidine (His - under the plane), and on the left side –
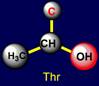
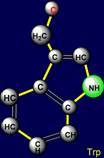
a pair threonine (Thr - above the plane) - tryptophan (Trp – under the plane)
are situated. Their properties are opposite in pairs: at the top are easy
side chains with the group C-OH, and at the bottom - heavy side chains with
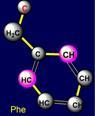
five-membered cycles. The side chain of cysteine (Cys - above the plane) forms pair with phenylalanine (Phe - under the plane) on the right side and the
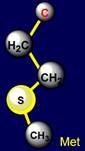
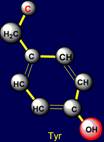
side chain of methionine (Met - above the plane) is paired with tyrosine (Tyr
- under the plane) - on the left side of the dodecahedron. Their properties
are also opposite: sulfur-containing side chains oppose the side chains of six-membered
rings. The side chain of aspartic acid (Asp - above the plane) forms a pair
with arginine (Arg – under
the plane) - on the right side of the dodecahedron. The side chains of
the pair have opposite properties: aspartic acid is donor of protone and can
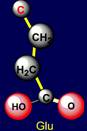
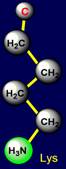
be negatively charged, and arginine – is acceptor of protone and can be positively charged. Similar properties appear in a pair of glutamic acid (Glu - above the
plane) and lysine (Lys - under
the plane), located
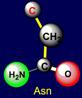
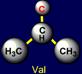
on the left side of the dodecahedron. The last pair of side chains on the right side is formed by a neutral asparagine (Asn - above the plane),
capable of forming hydrogen bonds, and valine (Val - under the plane),
nonpolar side chain which does not form H-bonds. It is obvious that their
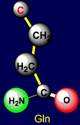
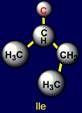
properties are also opposite. On the left side of the dodecahedron, a similar pair - glutamine (Gln -
above the plane) - isoleucine (Ile – under the plane). |
|
|
|
|
So, we were convinced, that the amino acids
connected by rotary antisymmetry, possess in pairs opposite properties. |
||||||
For the further
acquaintance with
the antisymmetry of the amino acids which
come to light by means of our model, pass in sections 4.2.1., 4.2.2., 4.2.4. or return to the
section 4.
Address
for connection: amino-acids-20@yandex.ru